|
Projects
Intracellular signaling mechanisms in the regulation of red
blood cell mechanical properties
Red blood cells (RBC) have long been considered as passive
transporters of respiratory gases, owing to their extremely high
hemoglobin concentration. Their mechanical properties are
important in fulfilling this function, however these properties
were also thought to be totally resulting from the special
material properties of these simple cells. New experimental
evidence gathered in the last few years started to change this
opinion. This project is exploring the molecular signaling
pathways within RBC that take part in the active regulation of
the ability of these cells to change their shape (i.e.,
deformability). This property is known to be the determinant of
blood flow under given hemodynamic conditions.
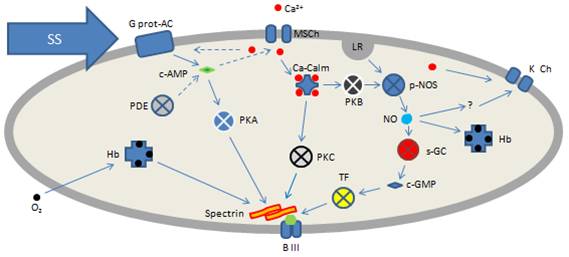
A unique mechanical response model is being used in
investigating the contribution of various intracellular
signaling cascades and target proteins contributing to this
regulation. The model is based on the slight but reproducible
increase in RBC deformability under constant shear stress in
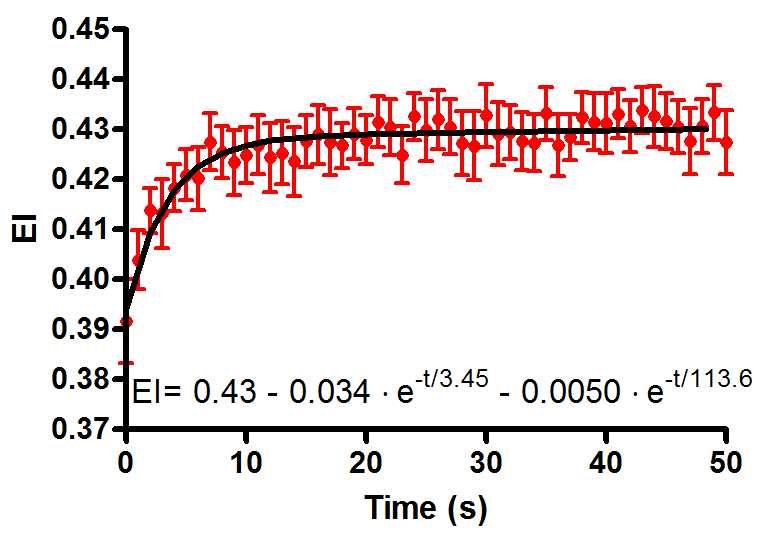
physiological range (1-10 Pascal). The figure on the right
demonstrates the increase in EI under 5 Pascal shear stress.
The experimental work includes the investigation of mechanical
response under the influence of various agents interfering with
the proposed signaling mechanisms (see below) and proteomic
approaches to identify the target molecular structures, in order
to understand the details of the regulatory mechanisms. The
previous research efforts to identify the role of nitric oxide
in RBC physiology and related mechanisms are also be combined
with the specific aims of this project. The project will be
expanded to include the regulation of RBC aggregability (e.g.,
by modulation of phosphatidylserine exposure on cell surface)
and oxygen affinity of hemoglobin by the same signaling
mechanisms. |
 |
Molecular and cellular
approach to erythrocyte aggregation and adhesion
The special way of
aggregation of erythrocytes (rouleaux formation) is an important
factor affecting a variety of in vivo hemodynamic processes that
influence blood flow and tissue perfusion. Despite the extensive
research the basic mechanisms for this physiological phenomena
are still not clearly understood.
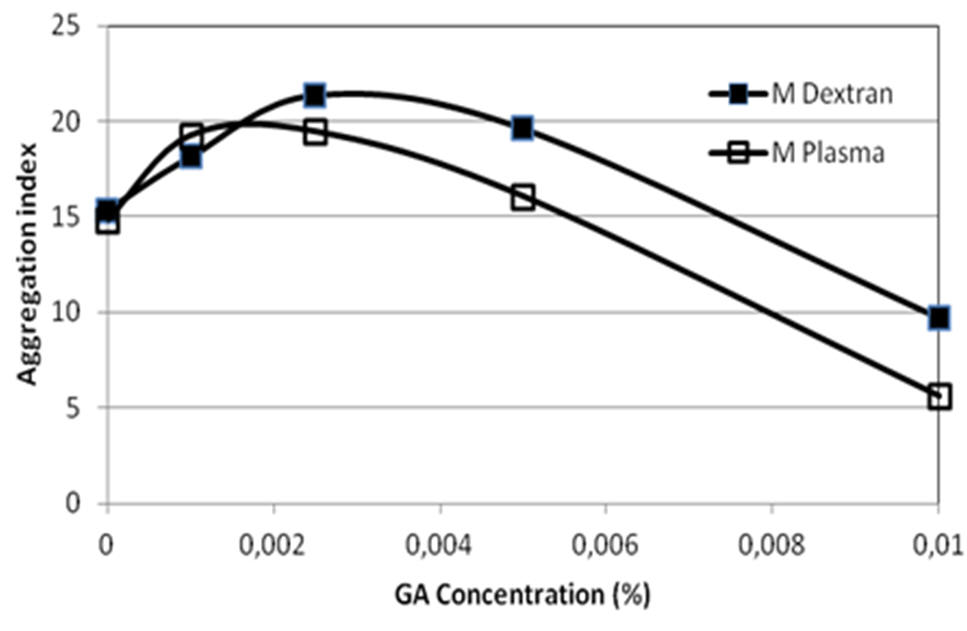
The main experimental approach is based on the models of altered
erythrocyte aggregability (i.e., the cellular properties
determining the degree of aggregation). The surface penetration
of aggregating macromolecules (i.e., high molecular weight
dextran) and adhesive forces between adjacent erythrocytes.
Treatment of human erythrocytes with very low concentrations of
glutaraldehyde (GA) is an example for such models (see the
figure on the right).
The project aims at investigating the surface phenomena that
determine the adhesive forces holding RBC together in aggregates.
Sophisticated techniques such as optical tweezers, optical
trapping microrheology, fluorescent/quantum dot labeling,
fluorescence resonance energy transfer and atomic force
microscopy are being used in the project.
|
 |